Resources

September 23, 2024
CF AMR Syndicate Announces First Awardees of £3m Collaborative Drug Discovery Programme
Cross-sector initiative provides funding and support to accelerate new treatments for lung infections in Cystic Fibrosis The Cystic Fibrosis Antimicrobial Resistance (CF AMR) Syndicate has today announced the first cohort of awardees of its Collaborative Discovery Programme (CDP) funding. The £3 million programme, funded by LifeArc, will support six early-stage novel antimicrobial projects over two […]

June 14, 2024
CF AMR Syndicate Achieves Gold Standard in Patient Partnership Index
The Cystic Fibrosis Antimicrobial Resistance (CF AMR) Syndicate has achieved the Gold Standard from the Patient Partnership Index (PPI). The PPI is the UK’s leading initiative in patient-centric communications and advocacy. Based on best practice, it evaluates the highest standards of partnerships between pharmaceutical, biotech and patient communities. The Gold Standard recognises the Syndicate’s demonstrable collaboration with […]

June 5, 2024
Diagnostic TPPs now out
The CF AMR Syndicate is delighted to announce that the diagnostic target product profile (TPP) guidance document is now available. TPPs are tools used by innovators that define criteria new diagnostic tests need to meet in order to fulfil specific patient and clinical needs. The acceptance or tolerance of a diagnostic test among people with […]

March 27, 2024
Harnessing the Value of Therapeutic TPPs for CF Lung Infections
In early 2022, the CF AMR Syndicate published our first-ever patient-focused therapeutic target product profile (TPP) guidance document for treating lung infections in CF. Building on this, the CF AMR Syndicate went on to announce their first funding call in March 2023, pledging £3M to support innovators and accelerate the development of novel antimicrobials to […]

July 13, 2023
Report from cross-sector collaborative workshop published
In October 2022, UKCFIB in collaboration with PIPE-CF held a joint cross-sector workshop, convening expertise on challenges and opportunities in preclinical testing of antimicrobials aimed at treating lung infections in CF. By bringing together expertise and perspectives from people with CF, industry, academia and the clinic, the workshop provided a platform to foster interdisciplinary knowledge […]
May 25, 2023
Strain guidance document published
The identification and selection of relevant microbial strains is an important consideration when testing new antimicrobials for CF lung infections. To guide the selection of appropriate strains for robust testing of new anti-infectives, Professor Eshwar Mahenthiralingham from Cardiff University alongside other experts in this area have produced a strain guidance document. The document, published open […]

March 16, 2023
£3M Funding programme launch
16 March 2023 Today (16 March 2023), Cystic Fibrosis (CF) Syndicate in Antimicrobial Resistance (AMR) announces a £3 million Collaborative Discovery Programme for drug discovery innovators to accelerate the development of new treatments for people with CF. Funded by medical research charity LifeArc, the programme will support approximately five collaborative projects that aim […]

September 28, 2022
LifeArc Joins CF AMR Syndicate as New Partner
LifeArc joins forces with Cystic Fibrosis Trust and Medicines Discovery Catapult to strengthen antimicrobial discovery efforts – pivotal partnership seeks to transform Cystic Fibrosis research LifeArc, a national medical research charity, is joining the Cystic Fibrosis (CF) Antimicrobial Resistance (AMR) Syndicate in a strategic partnership alongside Cystic Fibrosis Trust and Medicines Discovery Catapult (MDC). The […]

September 13, 2022
CF AMR Syndicate shortlisted for a Bionow award.
The CF AMR Syndicate was shortlisted for the Bionow AMR Award. At the award ceremony held in April this year, the Syndicate received a special mention for the way it partners with people with CF. More on the award ceremony including the full list of winners can be found here

September 6, 2022
CF AMR Syndicate at the 2022 UK CF Conference
The CF Trust’s Dr Paula Sommer and MDC’s Dr Beverly Isherwood presented on the CF AMR Syndicate at the UK CF Conference held in London earlier this year. Their talk, ‘Partnering for impact: the CF AMR Syndicate‘ was delivered in a session spotlighting initiatives focused on building the research landscape. The hybrid conference brought together […]
September 6, 2022
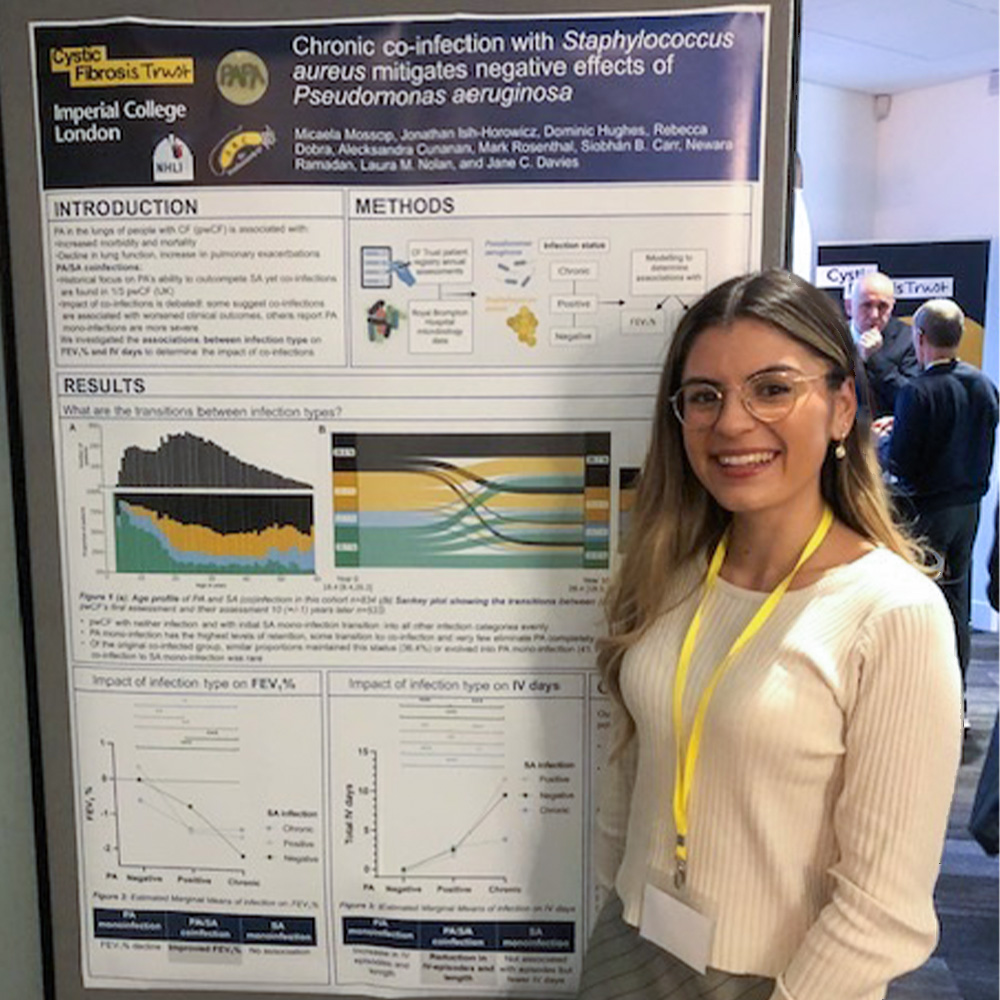
CF AMR Syndicate wins poster prize
The Inward AMR Mission to the UK took place on the 16-20th of May this year hosting 25 companies from the EU, USA and other parts of the world at three key AMR hotspots, Oxford, Alderley Park and Edinburgh. The Mission’s aim is to ‘help the global curbing of Antimicrobial resistance (AMR), by boosting collaboration […]
September 6, 2022
GOLD standard awarded to CF AMR Syndicate
Earlier this year, the Patient Partnership Index awarded the CF AMR Syndicate GOLD standard in recognition of the Syndicate’s demonstration of best practice in working with people with CF . Entrants to this year’s Index were assessed against the following six criteria: Engagement, Co-creation, Empowerment, Transparency, Innovation, and Impact. The Syndicate’s proactive involvement of people […]

June 13, 2022
CF AMR Syndicate: Lifting the Limits of Cystic Fibrosis Antimicrobial Development
Exploring the theme of ‘lifting the limits together’, this week (13-19 June) marks Cystic Fibrosis (CF) Week 2022. The event is a week-long opportunity to celebrate the courage and resilience shown by the CF community, whilst raising awareness and demystifying misconceptions surrounding the disease. CF is an inherited life-limiting multiorgan disease affecting over 10,800 people […]

August 10, 2021
Medicines Discovery Catapult and We Share Ventures collaborate in novel funding partnership
Medicines Discovery Catapult (MDC) and We Share Ventures have embarked on a joint venture, piloting an innovative funding model aimed at opening new funding opportunities for early-stage medicines discovery. For too long, accessing catalytic funding has been a challenge for medicines discovery start-ups focused on areas of clinical unmet need. This impact-driven revolving funding model […]