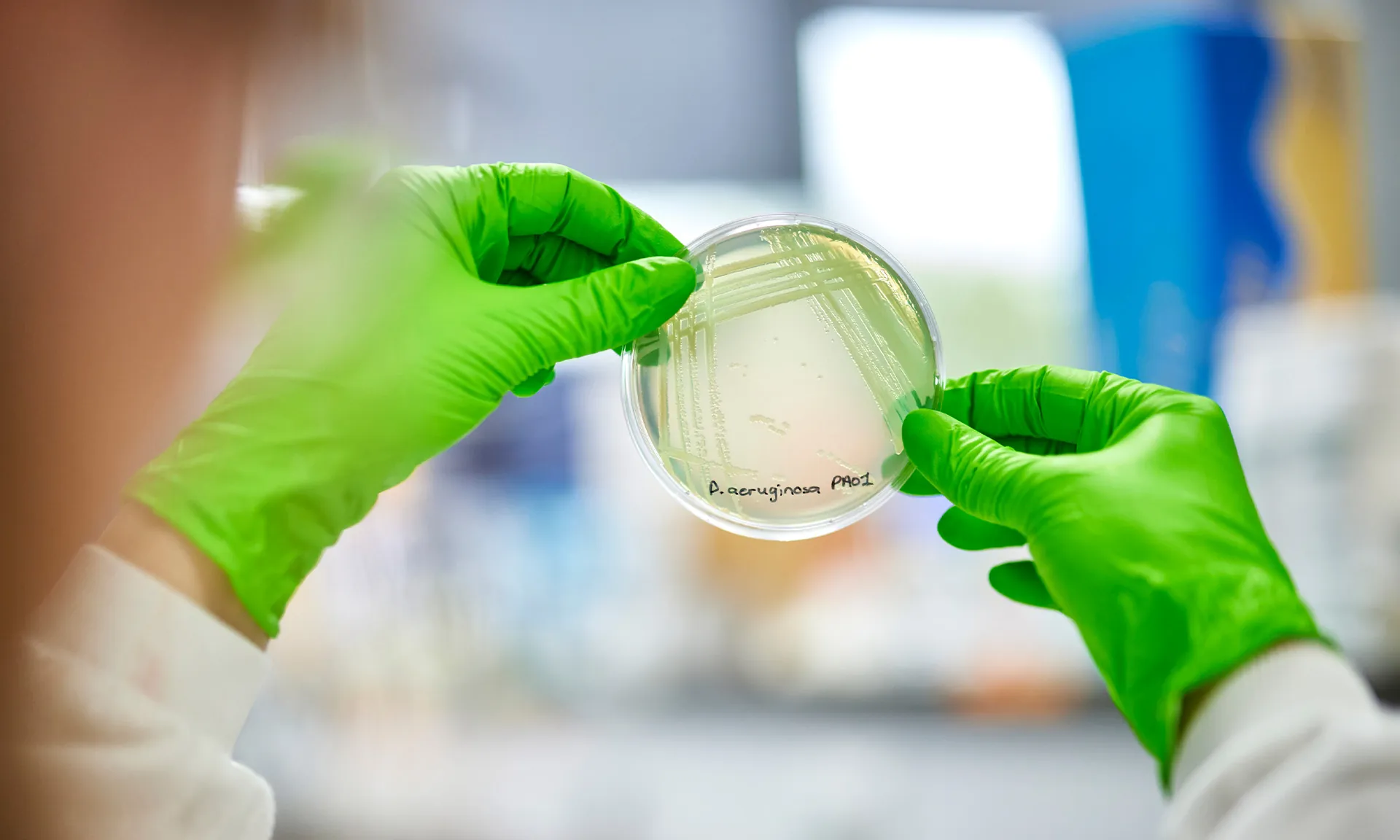
We caught up with Tom Barton, whose PhD is focused on developing laboratory models testing novel antimicrobial treatments in Cystic Fibrosis. Tom is working closely with Dr Jo Fothergill on PIPE-CF, an SRC co-funded by the Cystic Fibrosis Trust and Cystic Fibrosis Foundation. His paper, ‘Challenges and opportunities in the development of novel antimicrobial therapeutics for CF’ was recently published in the journal of medical microbiology.
Tom, can you tell us a little about the focus of your research?
My PhD focuses broadly on developing models of cystic fibrosis infection that can be used to test new antimicrobial treatments, so that we can more efficiently develop effective treatments for infection. Specifically, I focus on models that involve the host response, those that include not only pathogens, but factors involved in the human response to CF infection. An example of this includes incorporating, host cells and immune components in our models. We hope to optimise models that can accurately predict success of potential antimicrobial treatments, so that individuals with cystic fibrosis get more effective antimicrobials, faster.
In your paper, you identify collaboration within the CF community as an opportunity to be seized when it comes to antimicrobial drug discovery. Can you tell us why you think this is so important?
For CF antimicrobial research to be effective in improving the lives of people with CF, researchers must collaborate with those affected to identify priorities in future research. While eradication of infection is of primary concern, reducing unwanted side effects and lowering the burden of antibiotic regimens is also important – conclusions that can only be arrived at through collaboration with the CF community. To be effective in our response to CF infection, we must communicate with people with CF to ensure we remain focussed on maximally benefitting each aspect of their life.
You attended the cross collaborative PIPE-CF and UKCFIB joint workshop last year- what did you enjoy most about the workshop?
The workshop provided a forum for researchers, industry, policymakers and others to share views and ideas, which provided a unique perspective on CF research. This is invaluable, as it contextualises the research being carried out and gives clarity to the direction CF research will take. I think this is the most impactful part of the workshop.
What were your key take-aways from this event?
My key takeaways from the meeting include the opportunity to develop novel antimicrobial treatments for CF infection, and the focus on developing robust, representative models to improve the efficiency of developing new drugs. Novel antimicrobials, such as anti-virulence treatments that reduce the severity of infection could be instrumental in improving infection outcomes. Meanwhile, there is considerable opportunity for researchers to develop models that accurately mimic key aspects of the CF lung environment. Optimisation of our CF models will accelerate antimicrobial development, which I hope will lead to considerable improvements in infection outcomes in the future.
Are there any areas of research that you’re looking to collaborate in?
We have a strong focus on incorporating the host CF environment into our models, to gain more accurate insight into how potential antimicrobial treatments perform before they enter clinical trials. Any collaboration associated with host-pathogen models would be ideal.
How did you get into CF antimicrobial research, and why do you find it so interesting?
Cystic Fibrosis arises through a relatively simple mechanism, giving rise to a multifaceted problem with significant impacts on the lives of those with the disease. Understanding CF infection requires an understanding of how the host environment is altered and how bacteria and other pathogens live and communicate in the lungs. My PhD project is immensely interesting to me as it allows me to learn how these factors interact, and how we may model the environment to facilitate the development of CF antimicrobials. I hope the work we are doing may have a strong impact on CF antimicrobial development, and therefore on the wider CF community.