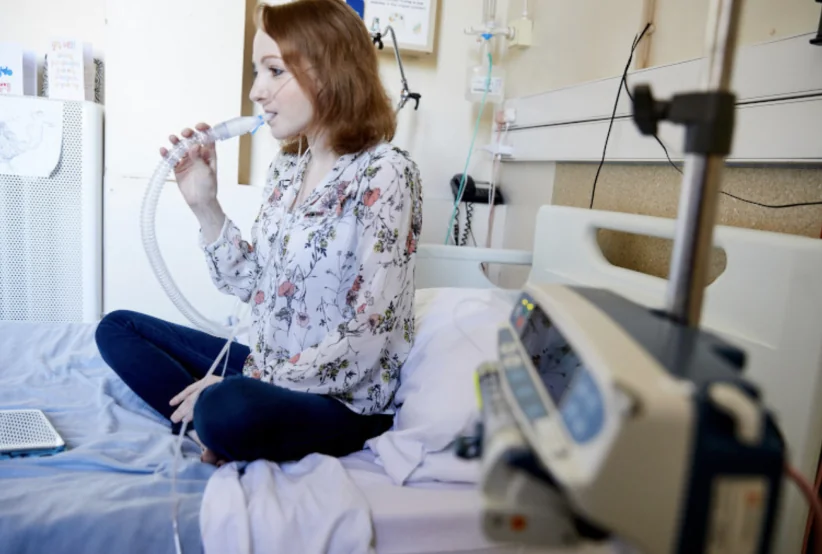
Biorepository
The UK CF Infection Biorepository (UKCFIB) was launched in Autumn 2021 to address a key challenge in CF antimicrobial discovery and development: the difficulty researchers face to access all the sample types they need for preclinical testing.
About the UK CF Infection Biorepository
Our vision is to build and strengthen access to high quality clinically relevant samples, data and expertise to enable researchers from academia and industry to identify and translate discoveries into new treatments for infections in people with cystic fibrosis, faster.
The biorepository runs as a coordinated national network of UK labs, each with existing collections and capability:
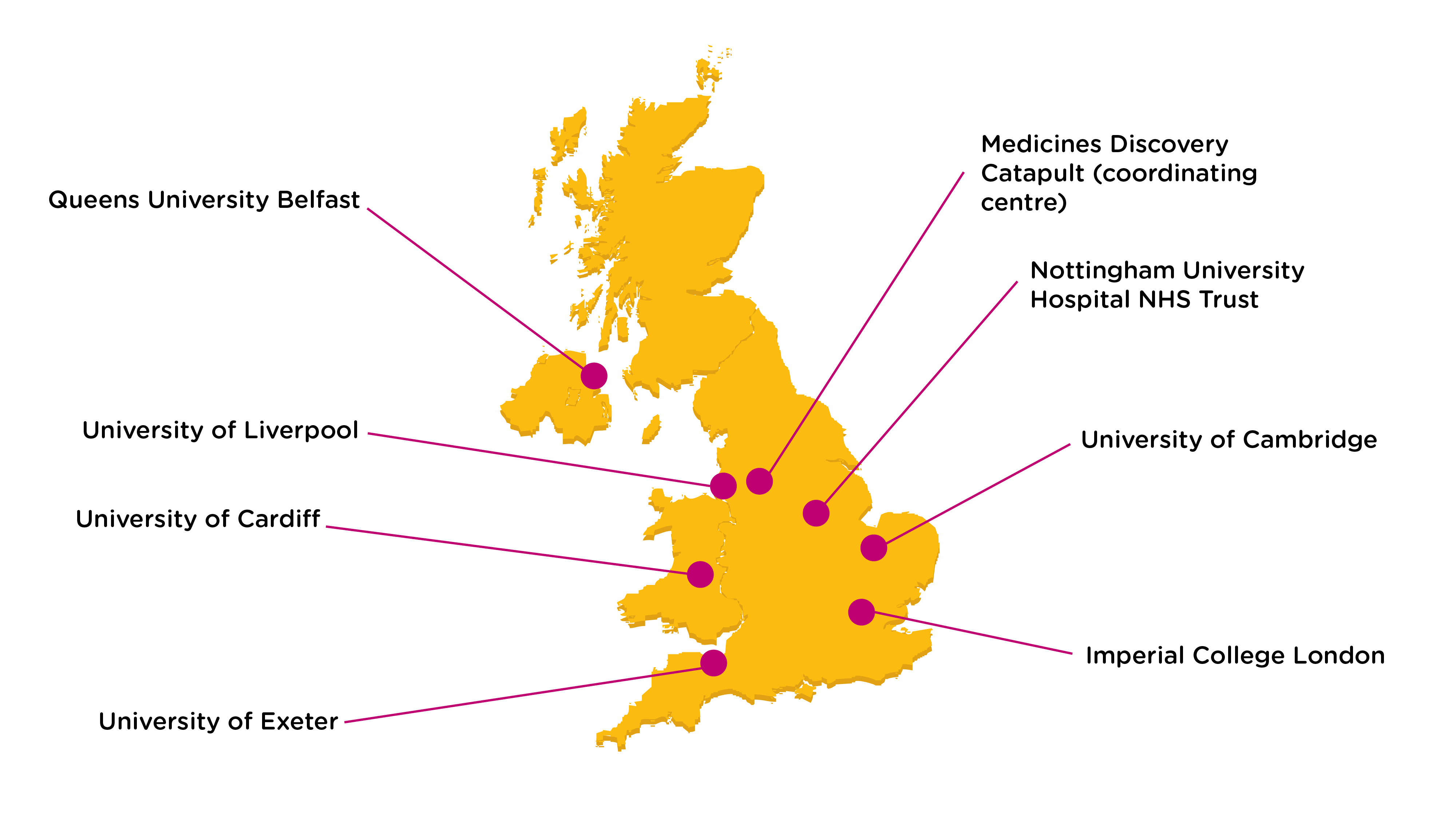
Accessing Samples and Capability from the UK CF Infection Biorepository
The biorepository supplies samples and engages in collaborative projects with academic and industry researchers in the UK and abroad.
Please complete the enquiry form to submit a request. If we think we are able to fulfill your sample request, we will set up a call with you to discuss your needs in more detail. We will then link you up with one or more UK centres that can fulfill your request.
All access requests are centrally managed by Medicines Discovery Catapult (MDC), which coordinates the UK network of participating laboratories. Requests for samples, data and capability are reviewed, approved and fulfilled by the relevant laboratory in the network with terms dependent upon the nature of the request.

UK CF Infection Biorepository Governance
UKCFIB is governed by a management team and receives ongoing strategic advice from an advisory committee.
The Biorepository Management Team (BMT) has responsibility for running the network. The group is made up of the principal investigators for each of the participating centres, the co-ordinating centre, The CF Trust and representatives of people with CF and their families. The group meets quarterly.
Members of the group are responsible for dealing with requests and work together to agree standard operating procedures, keeping abreast of operational and technical developments updating the operation as necessary.