Overview
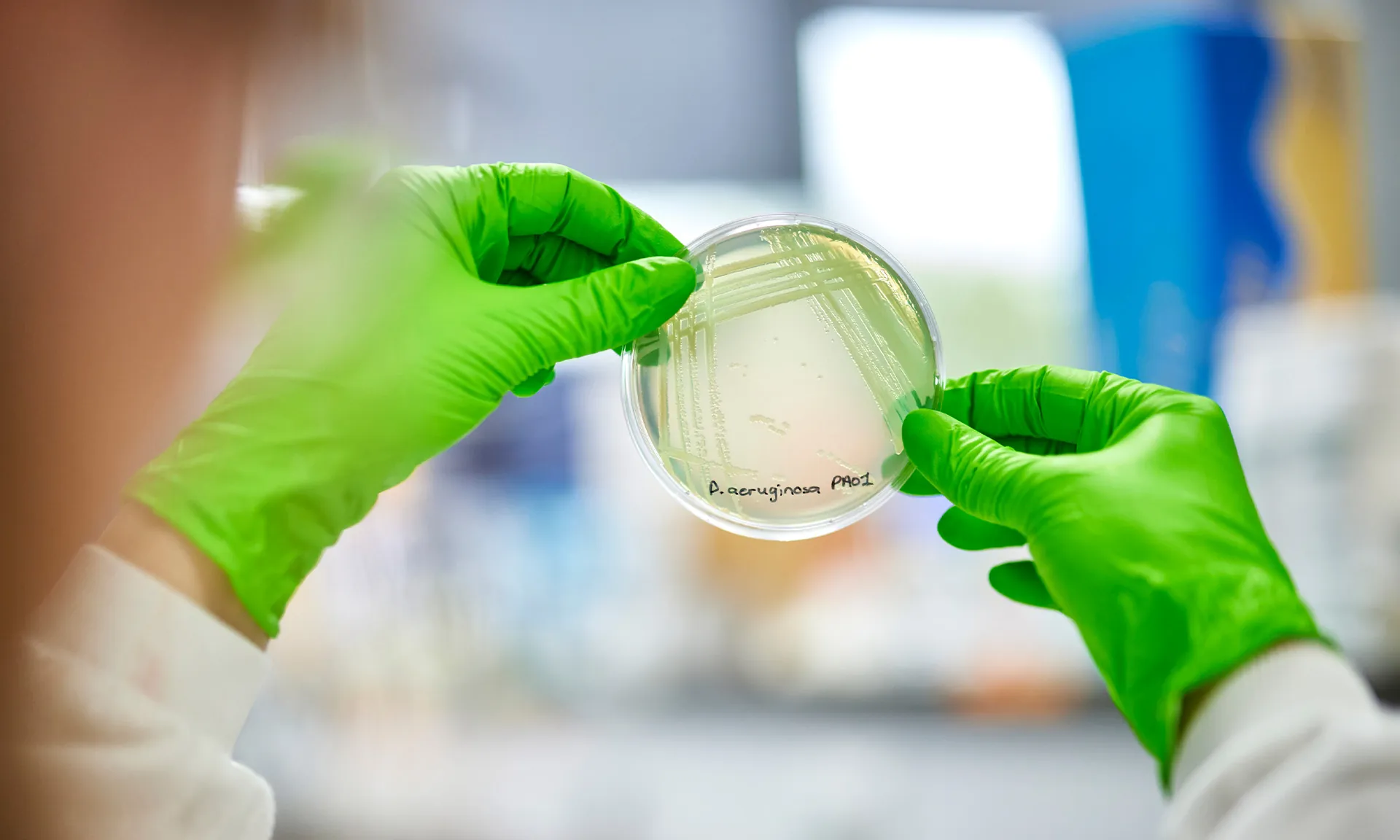
This Strategic Research Centre (SRC) will lay important foundations for the best way to accurately test the effectiveness of new antibiotic medicines for CF pulmonary infections. The SRC is co-funded between the Trust and the CF Foundation in the United States and is led by Dr. Jo Fothergill at the University of Liverpool. PIPE-CF involves an international consortium of 7 Universities and 2 NHS Trusts.
The aim of this Strategic Research Centre is to make it easier for researchers to develop new antibiotics specifically designed to treat pulmonary exacerbations in CF. They will do this by creating and checking new methods to test the effectiveness of new antibiotics. These new methods could then be used by university and industry-based researchers around the world.
Background
There are two steps for the development of new medicines in the laboratory. The first step is finding that a chemical or compound could have potential benefit as a new medicine. Sometimes this stage is known as the ‘discovery phase’. The second step is converting that promising chemical into a new medicine. This is usually known as the ‘pre-clinical phase’ of development of a new medicine. If the results of the pre-clinical phase of testing are positive, the medicine will go on to be tested in clinical trials.
When medicines are approved by regulators for use, the regulators use information from clinical trials and ‘pre-clinical’ tests to help make their decision. However, standardised methods for the testing of new antibiotics to treat CF pulmonary infections have not been developed or approved by the regulators. This represents a significant hurdle for researchers in terms of time and cost.
Aims of the project
The Strategic Research Centre will develop and check a new set of pre-clinical tests specifically to assess the effectiveness of new antibiotics for CF. The aim is that these new tests will make it quicker and easier for researchers to develop new CF medicines in the future.

The SRC research will include investigations into the following:
01
Choosing the most representative ‘strains’ of bacteria to include in the tests
02
Developing tests to mimic how different pathogens work together in the CF lung. Many different pathogens reside within the CF lung and they can all influence each other- this is something that drug developers should keep in mind
03
Predicting how individuals with CF would respond to infection, in laboratory tests. This will be done by looking at how new therapies could work in harmony with a person’s immune response
04
Adapting tests to assess the best dose of the new CF medicines – There are no lab tests to specifically work out the best dose to use for new antibiotics in CF. Researchers will adapt a novel method for assessing the dose for antibiotics for other conditions to mimic the environment within the CF lung.
Join our network
Join our network and be part of a collaborative force driving groundbreaking cystic fibrosis antimicrobial research.
Contact the PIPE CF Team
If you would like more information about PIPE CF, please get in touch.