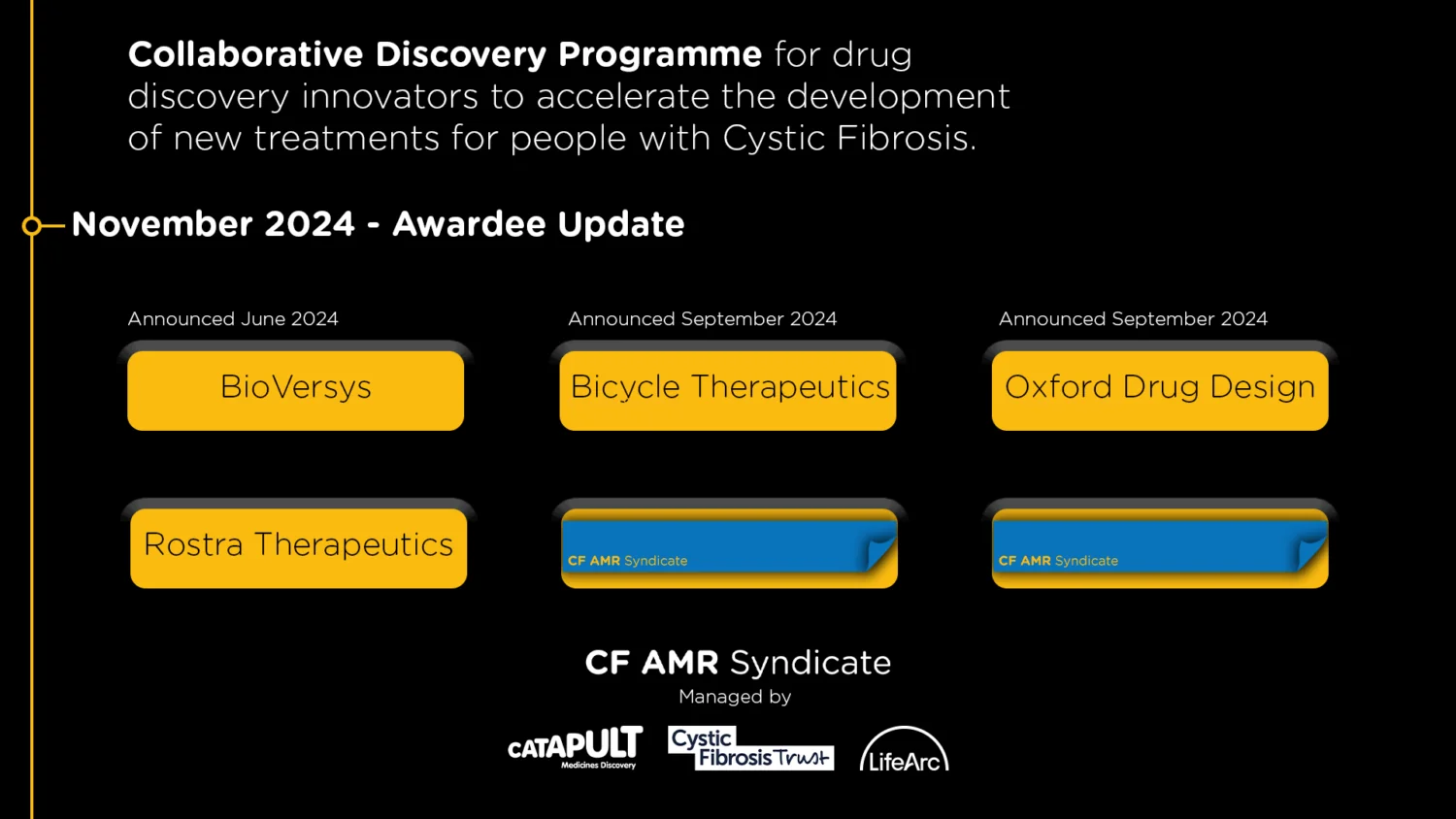
In October 2022, UKCFIB in collaboration with PIPE-CF held a joint cross-sector workshop, convening expertise on challenges and opportunities in preclinical testing of antimicrobials aimed at treating lung infections in CF. By bringing together expertise and perspectives from people with CF, industry, academia and the clinic, the workshop provided a platform to foster interdisciplinary knowledge exchange, stimulating thinking around focus areas of innovation needed to optimise the tools and resources available for preclinical CF antimicrobial medicines discovery. The interactive event drew over 50 delegates, including international participation.
Some key takeaways from the cross-sector workshop included:
- The need to consider the priorities of people with CF at the early stages of drug discovery- guidance documents like the therapeutic TPPs capture some of these priorities. Mode of drug delivery, minimising toxicity and reducing the risk of drug-drug interactions were also highlighted as key considerations.
- The lack of relevant preclinical models that better predict clinical success, are adapted to support the diversity of therapeutic modalities emerging and tailored to meet regulatory requirements was highlighted as a key challenge. There was an emphasis on drug developers to engage early with people with CF via organisations like CF Trust, clinicians, investors and regulators to inform their R&D programme and data package.
A detailed report discussing outputs from this workshop is available here.