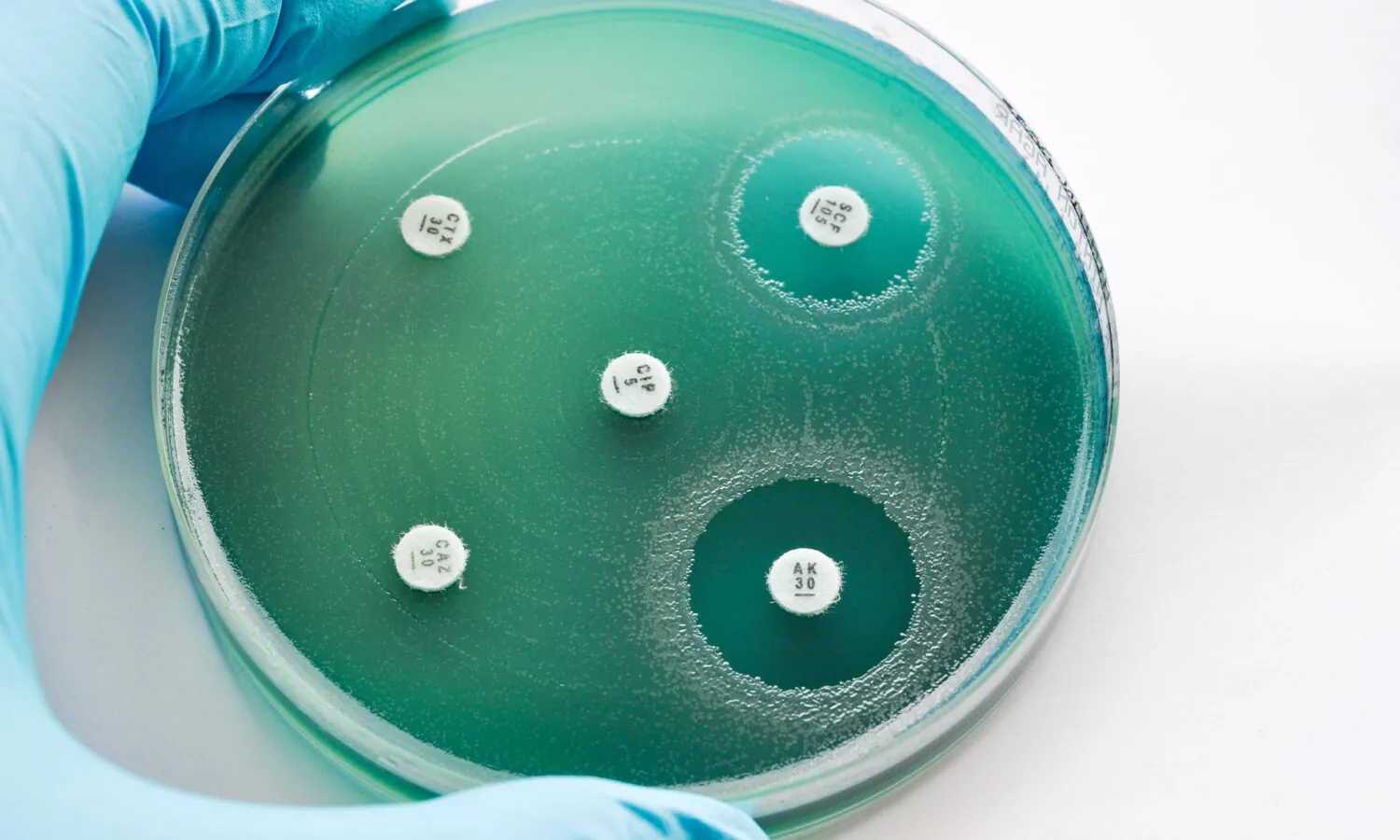
The CF AMR Syndicate is delighted to announce that the diagnostic target product profile (TPP) guidance document is now available. TPPs are tools used by innovators that define criteria new diagnostic tests need to meet in order to fulfil specific patient and clinical needs.
The acceptance or tolerance of a diagnostic test among people with Cystic Fibrosis is paramount to its successful use in both community and healthcare settings. The CF AMR Syndicate, together with the NIHR Newcastle HealthTech Research Centre (HRC), have worked extensively with the CF/AMR community, engaging with over 150 different stakeholders, including people with CF and their loved ones, to define the unmet diagnostic needs. The guidance document is intended to support and advance diagnostic R&D, focusing efforts where they are needed the most. Get in touch with us if you are looking for collaborators or have a project/programme relevant to the diagnostic needs identified through this work.
Request your free copy here.