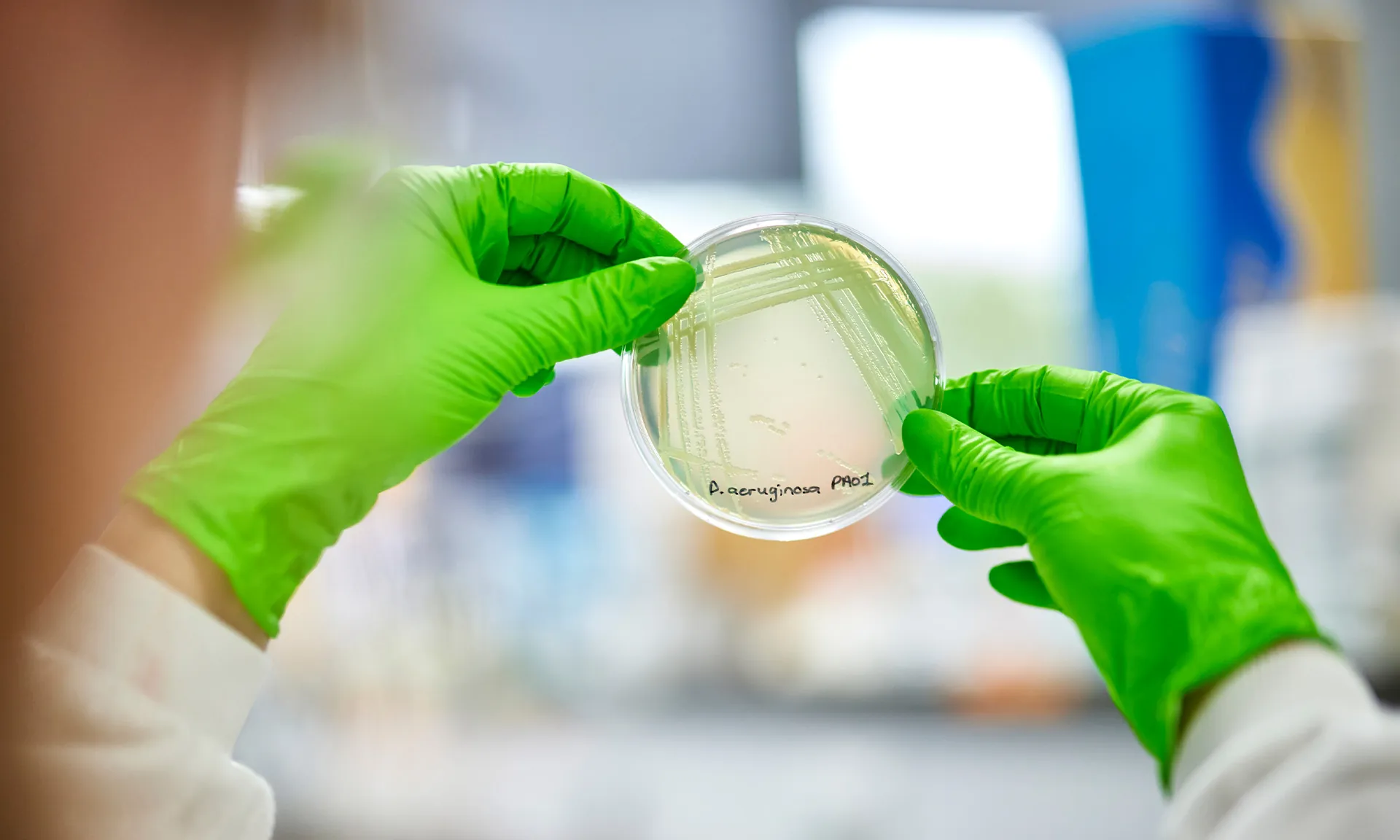
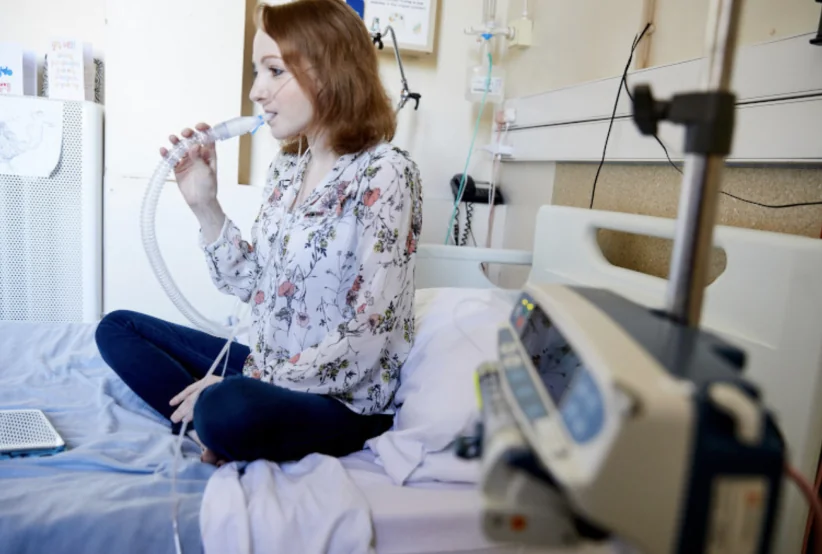
Monday 4 August, is NTM Awareness Day, an important opportunity to raise awareness about nontuberculous mycobacterial disease (NTM). This condition continues to impact people with cystic fibrosis (CF) and others living with chronic lung infections.
NTM infections are notoriously difficult to manage, and current treatment options are limited. More innovation is urgently needed to help drive the pipeline.
Through the Collaborative Discovery Programme, the CF AMR Syndicate is privileged to support BioVersys, an SME tackling this urgent unmet need by advancing its asset targeting NTM.
We spoke to Dr Marc Gitzinger, CEO at BioVersys, to find out more.
Please tell us about BioVersys and its mission in the field of infectious diseases.
BioVersys is a company I started directly from my PhD thesis at the Swiss Federal Institute of Technology. We are fully focused on antibiotic resistance and infectious diseases. The company has grown, and we have a rich pipeline, focused on the high unmet medical needs in the infectious disease and antimicrobial resistance (AMR) space.
We recently took the company public on the Swiss stock exchange. We have two clinical assets: one is entering phase three for resistant Acinetobacter infections in the lung, and the second is a project against tuberculosis, which is in phase two clinical development, partnered with GSK. Our preclinical pipeline has two assets: one on Staphylococcus aureus, and very importantly, the second is focused on NTM.
Can you share more about your current project and how it aims to support people living with cystic fibrosis (CF) and NTM lung infections?
Our NTM project is based on one of our more advanced pipeline projects, which uses ansamycin chemistry (a group of antibiotics produced by certain actinomycetes strains, which display anticancer, antibacterial, and antiviral activities). It has a few family members from that group, including well-known drugs like rifampicin and rifabutin.
When we started working on this family, we realised that it was probably underexplored in terms of chemistry and that additional work needed to be done around the core of that chemical structure and also the final drug target: the RNA polymerase.
We further optimised within the family to overcome preexisting resistance, particularly in the NTM species. We also optimised the PK/PD profile (improved how the drug is absorbed, distributed, and works in the body), including the potential for drug-drug interactions to reduce the CYP induction (an increase in enzyme activity, which leads to faster metabolism of drugs) that is intrinsic to the family.
We went really specific from a rather well-known starting point to overcome some of the issues, and we had the chemical means to do so. That means that we believe we have an asset that not only shows very good efficacy (or effectiveness) but that we also know a lot about the safety profile already around it. By delivering a new molecule or drug for patients that is not only efficacious but also has a good safety profile, you provide better outcomes for patients.
What has your experience of the CDP been like, and how has the Syndicate helped move your research forward?
We were one of the first projects selected by the CF AMR Syndicate, and we have had a wonderful experience. That’s not only speaking about the obvious funding provided, but also from the scientific expertise. Our team has enjoyed the interactions a lot. We got a lot of inputs on the scientific level, but also, with assets like additional clinical isolates from NTMs, etc. We have had access to resources that are not so easy to get to, as a smaller company. We are very happy with this collaboration.
How has the Syndicate helped you access patient insight?
As a drug developer working with chronic and multifactorial diseases, you should understand the everyday challenges patients face and all the therapies they must take.
It is important that your molecule is not only efficacious against the bacteria being treated but also offers a possibility of improved quality of life. That means providing convenience in how the patient takes the drug to encourage regular use with minimal side effects.
In that sense, it’s great that the Syndicate offers the possibility to not only listen to patient voices, but also to be connected with both patients and physicians, to learn directly from them.
How does working collaboratively with the Syndicate differ from more traditional drug development approaches?
Most traditional drug development approaches focus very strongly on the science, particularly in the early stage of drug development, where it’s about the new target or the target that you address, the physical and chemical properties and so forth.
You very easily risk getting lost in the science and optimising only that, but in doing so, you are forgetting the everyday life of your different users: it is important to consider the perspective of the physician who will prescribe the drug as well as the patient.
That’s fairly unique because, in early-stage projects, scientists might forget that picture. It makes a big difference for the patient to see that they not only have an effective drug, but ideally also one that best suits their reality.
Do you have a message about your commitment and progress that you would like to share with the CF and NTM community on NTM Awareness Day?
At BioVersys, we are dedicated to providing solutions for people affected by NTM disease, cystic fibrosis, and COPD. NTM is difficult to treat overall and requires long-term treatment. We are fully committed to developing molecules that are hopefully very tolerable and highly efficacious in treating these bacteria and giving patients back their quality of life.
We recently announced that we are partnering our NTM asset with Shionogi. We are looking forward to working with them to de-risk the development of this program further and expedite the development towards clinical trials for our NTM asset.
It’s a very good trajectory because the program was in its early stages when we joined the CF AMR Syndicate. We advanced it through the early development phase and have already been able to attract a large pharmaceutical partner in Shionogi. Now, we are focused on ensuring that this program has the best chance of reaching clinical development and patients.
What the Syndicate is doing with connecting us to the community and patients is great. This is much more established in CF, and I wish we had more of that in the acute infection space.
Initiatives like the Syndicate are super important, and so I’m really glad to be part of this. We develop these molecules in the lab, and it feels so early – more than ten years away from anything real. But in the end, you develop it for the patients. You should never forget about that.
Watch the full interview with Dr Marc Gitzinger: