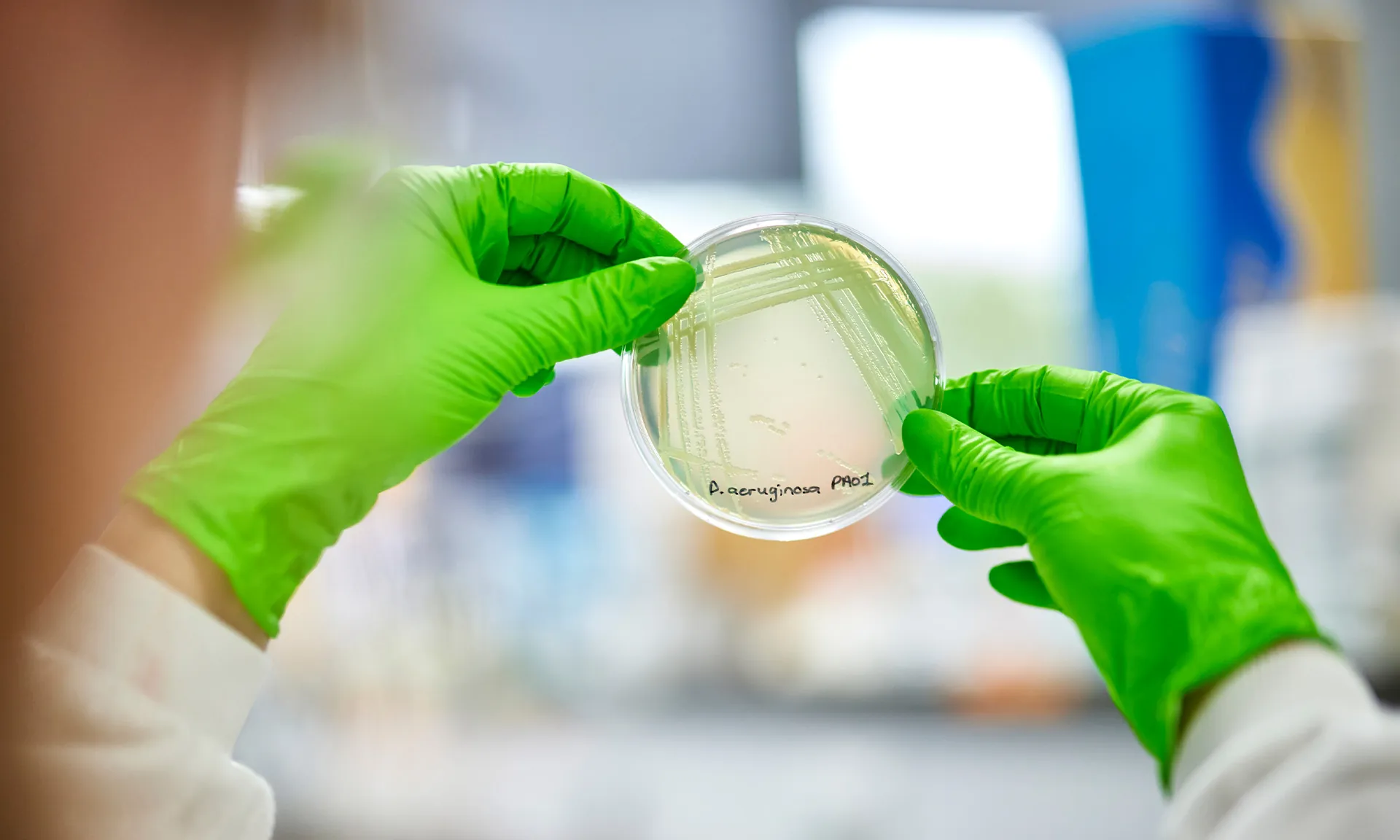
Non-tuberculous mycobacteria, or NTM, are a group of bacteria that cause lung infections that can be very difficult to treat. Although rare, they are more likely to affect people living with long-term lung conditions such as Cystic Fibrosis (CF). Because NTM infections can be challenging and complicated to treat, the quality of life of those affected is often impacted.
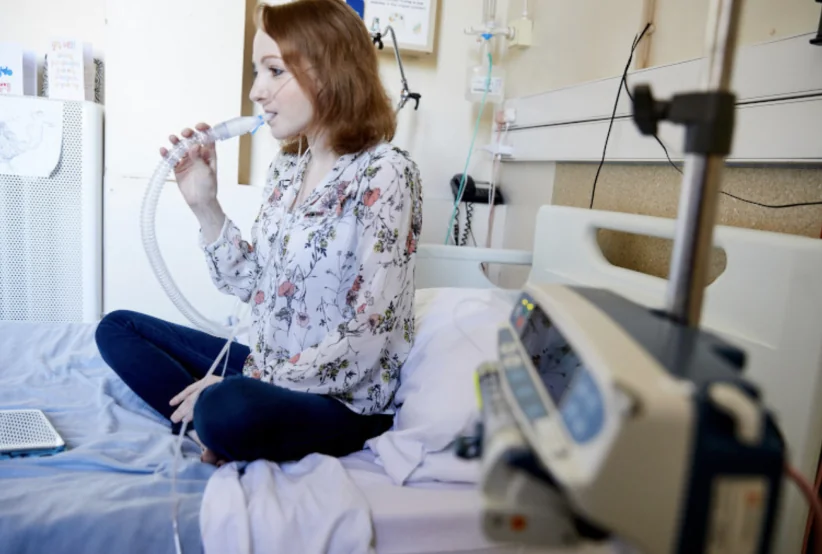
Michelle’s story brings to life some of the challenges surrounding the detection and diagnosis of this group of bacteria.

Waiting for the results
Current methods for detection of NTM involve standard culturing techniques from airway samples which are time consuming, due to the slow growing nature of the organisms, and this can take upwards of three months, leading to delayed diagnosis. In addition, obtaining information on which correct antibiotics to use is slow and isn’t always reliable.
Whilst routine monitoring for infection was something that Michelle was more than familiar with as part of her ongoing management of CF since her diagnosis as a baby, in August 2022, Michelle was told she had tested positive for and grown a bacteria called Mycobacterium abscessus (M. abscessus), a type of NTM, for the first time.
“From my experience, it takes around eight weeks to grow M. abscessus in the lab. This is a frustratingly long time to wait to receive a diagnosis and understand the sensitivities.
It’s such a long time [to wait for results] — it doesn’t feel like a system that’s set up for modern-day medicine.”
Reliability of testing
To add further complexity, the detection process for NTMs using culture can be unreliable. As these organisms can be found in the environment, positive detection of NTM from an airway sample is not always indicative of NTM infection. For this reason, multiple positive samples are needed for a formal diagnosis of NTM.
Once Michelle started treatment for the infection, she had a large number of consecutive tests over nine months which reported as negative for NTM. However, she later received another positive result and explains how this impacted her.
“As a patient on such an aggressive treatment plan, you are constantly hoping that the treatment is working. I knew of the potential long-term and irreversible side effects of the medication, so requesting regular updates and providing regular samples to monitor growth helped me to navigate and justify those risks. However, the length of time that it takes to grow the bacteria means that by the time you get the results, those result are already out of date. I had a period of nine months, at the beginning of 2023, where every sample came back negative (11 negative results in a nine-month period). The twelfth sample was positive. That process allowed me to build up hope, which made it even harder learning it had returned.”
Additional impacts
“Finding out I had the infection again was a blow. Because of my genetic make-up, I am not currently eligible to take the modulator drug, Kaftrio (a highly effective drug which treats cystic fibrosis). I had closely followed the development of genetic therapies for CF and I had hoped to become involved in the trial for one of those treatments. At the time that I learned of the positive result, I had already reached out to one trial coordinator.
I knew that NTM could limit your options in terms of enrolling on trials, but I had hoped by the time the trial had completed the set-up stage, I would have the required period of clear results. As it turned out, I was taken back to square one. That had a substantial psychological impact on me.
Luckily, I can advocate for myself, and I have a great CF care team, with doctors who listen to me, and take on board my wishes. I started a fresh and even more aggressive treatment plan and I have recently had three consecutive negative results. I’m almost reluctant to voice that out loud, though. Having been here before, I’m aware that this doesn’t necessarily mean anything. I don’t really know how to feel or what to think, other than, surely there should be a better process than this?”
Addressing the challenges associated with the diagnosis and management of NTMs
The improvement of diagnosis and treatment for NTM infections has huge potential to improve the lives of people living with CF and other lung conditions.
The CF AMR Syndicate, together with the Newcastle NIHR HealthTech Research Centre, has developed a suite of Target Product Profiles (TPPs) for CF lung infection diagnostics which include a TPP focused on diagnosing NTM lung disease due to the limitations of current testing. These TPPs provide guidance for drug and diagnostic developers on which key characteristics and requirements are necessary to successfully address the needs and priorities of people living with CF.

Get involved
There are multiple ways you can support us to improve the lives of people living with NTM infections.
- Get in touch if you would like to share your own personal experience with NTM.
- Visit our website to find out more about how the CF AMR Syndicate is supporting research in this area.
- Request a copy of our TPPs to see where you can support identified unmet patient needs.
- Join our CF AMR network, home to cross-sector collaboration, with the expertise and drive required to accelerate CF antimicrobial development.
- Working on a project linked to some of the topics discussed in this article? Get in touch to see how we can support you.