Advancing Preclinical Phase Projects for
Cystic Fibrosis Lung Infections
The CF AMR Syndicate Collaborative Discovery Programme (CDP) is advancing an exciting and diverse pipeline of preclinical phase projects for Cystic Fibrosis (CF) lung infections. Our £3 million (GBP) programme (provided by LifeArc) is supporting six early-stage drug discovery projects aligned with the unmet needs identified in our Therapeutic Target Product Profiles (TPP) for CF lung infections.
CDP offers a unique collaborative approach to project development and delivery. As well as funding, our awardees benefit from additional collaborative support, increasing the probability of successful outcomes.
Support through the early development phases to generate essential data packages will position these projects to attract onward funding and investment for further development.

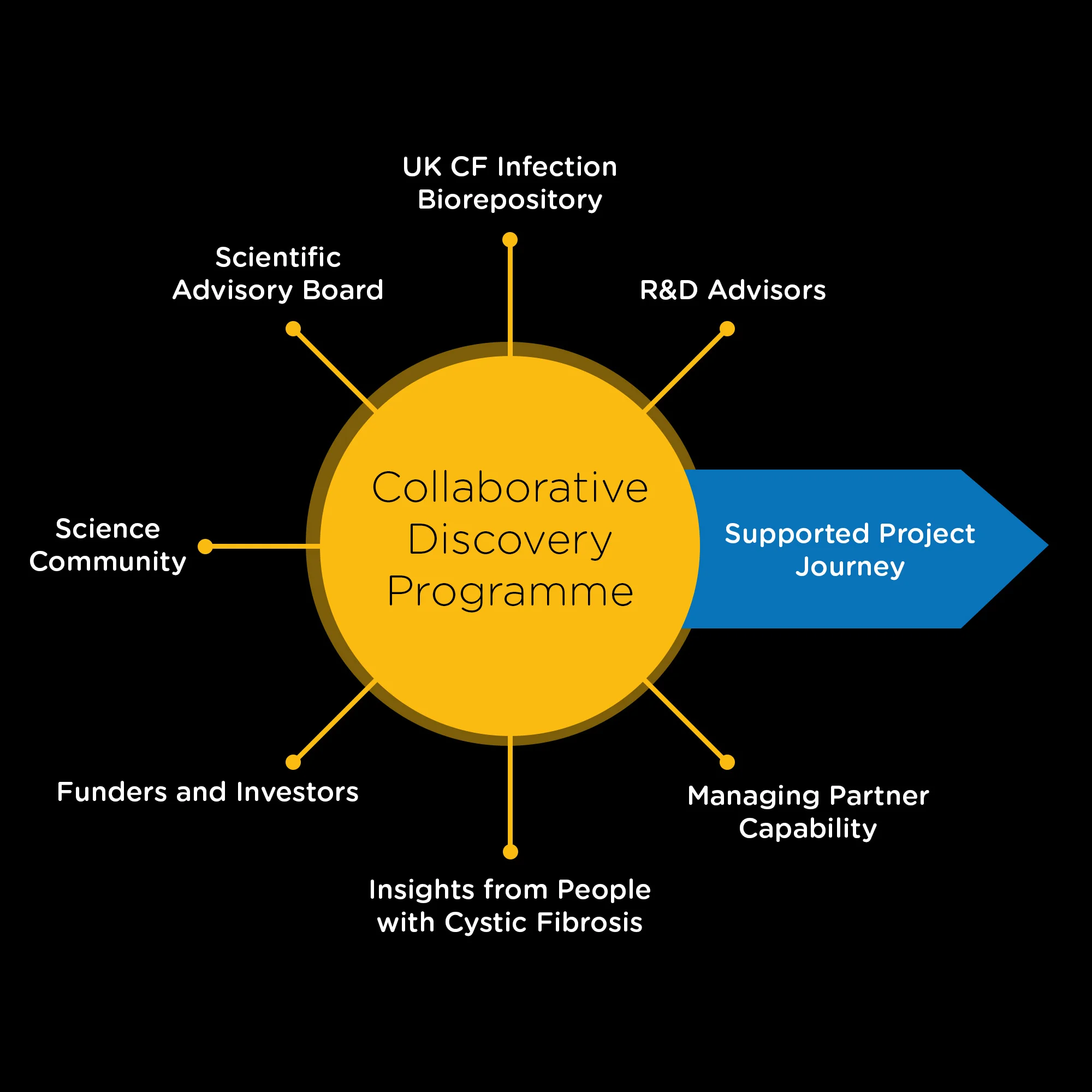
More than just funding
Collaboration at the Centre
- Collaborative support – overcoming barriers together
- Provision/access to CF AMR Syndicate resources
- Drug discovery and disease area insight and advice
- Support to develop actionable project plans aligned to TPP
Our project pipeline
Project Summary
BioVersys’ Non-tubercular Mycobacteria (NTM) preclinical program is derived from the company’s proprietary Ansamycin Chemistry platform. The BioVersys’ research team is developing a novel and highly potent best in class, broad-spectrum anti-NTM ansamycin, suitable for oral or inhalation therapy that is devoid of cross-resistance with other therapeutic classes and does not show any significant potential for drug-drug interactions.
Company Summary
BioVersys AG is a multi-asset, clinical stage biopharmaceutical company focused on identifying, developing and commercializing novel antibacterial products for serious life-threatening infections caused by multi-drug resistant (“MDR”) bacteria. Derived from the company’s two internal technology platforms (TRIC and Ansamycin Chemistry), candidates are designed and developed to overcome resistance mechanisms, block virulence production and directly affect the pathogenesis of harmful bacteria towards the identification of new treatment options in the antimicrobial and microbiome fields. This enables BioVersys to address the high unmet medical need for new treatments against life-threatening resistant bacterial infections and bacteria-exacerbated chronic inflammatory microbiome disorders. The company’s most advanced research and development programs address nosocomial infections of Acinetobacter baumannii (BV100, Phase 2), and tuberculosis (alpibectir, Phase 2a, in collaboration with GlaxoSmithKline (GSK) and a consortium of the University of Lille, France). BioVersys is located in the biotech hub of Basel, Switzerland.
Project Summary
Bicycle have developed proprietary technology, known as bicycle molecules, which are fully synthetic short peptides constrained with small molecule scaffolds that stabilize their structural geometry, resulting in high binding affinity and selectivity. The team are aiming to develop a new class of antibiotic targeting P. aeruginosa infections, with a focus on the suppression of chronic infection in pwCF in line with the CF AMR Syndicate Target Product Profiles.
- The treatment durations suggested for new therapeutics
- The use of CF animal models and traditional infection rodent models in preclinical studies
- The use of patient-reported outcomes (PROs) as primary clinical trial endpoints in clinical studies
Company summary
Bicycle Therapeutics is a clinical-stage pharmaceutical company developing a novel class of medicines, referred to as Bicycle® molecules, for diseases that are underserved by existing therapeutics. Bicycle molecules are fully synthetic short peptides constrained with small molecule scaffolds to form two loops that stabilise their structural geometry. This constraint facilitates target binding with high affinity and selectivity, making Bicycle molecules attractive candidates for drug development.
Our focus is an early stage drug discovery to produce robust prototype molecules that have all of the properties required to ultimately become a highly effective and safe new medicine to treat infections in CF patients.
Project Summary
Oxford Drug Design is developing a highly innovative small molecule approach targeting leucyl-tRNA synthetases in bacteria. These novel chemotypes represent a new class of antibiotics with no cross resistance with existing antibiotic classes.
Company Summary
Oxford Drug Design, an advanced spinout from Oxford University, is an innovative biotechnology company discovering and developing novel therapeutics supported by pioneering computational methods.
We design innovative drugs with differentiated modes of action against a wide range of challenging diseases with high unmet medical need seeking superior patient outcomes.
Based on the unique integration of our dual core competences in aminoacyl-tRNA synthetase enzymes and distinctive AI/Machine Learning methods we are able to achieve rapid and effective outcomes in developing novel treatments. This distinctive drug discovery platform has been validated by the rapid discovery of novel therapeutic candidates with highly innovative chemical scaffolds and modes of action.
Project Summary
Through this project, and subsequent preclinical and clinical work, we aim to develop a novel S-MGB drug that will eradicate fungal lung infections in CF patients. This will address the need for better management of infections in people living with CF by addressing their serious invasive fungal diseases that are hard to eradicate due to the increase in antimicrobial resistant infections.
The goal of the project is to evaluate a short series of diverse S-MGBs as antifungal agents in the context of CF so that the risks associated with this class of compound are identified and made manageable through the new data that will be obtained. The successful outcome of this project will lead to a medicine that will address the threat of fungal lung disease in CF patients.
Company Summary
Rostra Therapeutics is a new company founded in Glasgow to develop a therapeutic technology developed at the University of Strathclyde. Bringing together a highly experienced science, commercial and operational team, Rostra’s mission is to develop a novel platform of molecules, the 2nd generation Strathclyde Minor Groove Binders (S-MGBs), into medicines to address serious infectious disease. Various S-MGBs have demonstrated efficacy against many different pathogens including fungi, viruses, bacteria and parasites. The initial focus of Rostra Therapeutics will be on developing specific S-MGBs for the treatment of serious and invasive fungal infections. This represents an area of significant unmet clinical need with over 2.2 million deaths from fungal disease each year globally.
Project Summary
Glox’s proprietary protein-based antimicrobials (engineered bacteriocins) are highly potent precision therapeutics that kill the targeted pathogen but leave the wider microbial community intact. This targeted approach has the potential to retain colonisation resistance afforded by host microbiota, reducing subsequent drug-resistant infections that are a frequent consequence of conventional broad-spectrum antibiotic use. Glox will utilise their proprietary bacteriocin engineering platforms to develop a targeted therapy for P. deruginosa lung infection in people with CF with the aim of reducing the burden of standard antibiotic therapy, the frequency of pulmonary exacerbations, and ultimately reducing the rate of decline in lung function and prolonging life.
Company Summary
Glox Therapeutics are developing precision antibiotics to combat antimicrobial resistance. Our goal is to create potent, species-specific antibiotics that, unlike conventional antibiotics, do not harm the host microbiome. We are producing engineered bacteriocins from our proprietary platform technologies to target Gram-negative pathogens, including P. aeruginosa.
Project Summary
Early-stage compounds have demonstrated potent activity against drug-resistant bacteria that cause CF-related lung infections, with low resistance potential and rapid bacterial killing. This project aims to optimise these compounds for preclinical studies and evaluate the potential of these compounds as treatments for CF-related lung infections.
About King’s College London
King’s has an outstanding reputation for world-class teaching and cutting-edge research, and its Institute of Pharmaceutical Science has a strong track record in discovering and developing medicines. In the 2021 Research Excellence Framework (REF), King’s maintained its sixth position for ‘research power’ in the UK. King’s has also been rated third amongst multidisciplinary institutions for impact, with 67.8% of its research impact rated outstanding.
About UKHSA
UK Health Security Agency (UKHSA) prevents, prepares for and responds to infectious diseases, and environmental hazards, to keep all our communities safe, save lives and protect livelihoods.
We provide scientific and operational leadership, working with local, national and international partners to protect the public’s health and build the nation’s health security capability.
More on the Call Scope
This first funding call sought to identify project proposals with the potential to address needs identified in our therapeutic TPPs in particular:
Antimicrobials focused on Pseudomonas aeruginosa infections as 1) a maintenance therapy to suppress chronic respiratory infection or 2) treatment of acute pulmonary exacerbations linked to Pseudomonas aeruginosa infection.
Antimicrobials focused on Mycobacterium abscessus infections as 1) a maintenance therapy to suppress chronic respiratory infection or 2) treatment of acute pulmonary exacerbations linked to M.abscessus infection or 3) eradication treatment. Also in scope, treatments targeting ‘fast-growing’ NTMs of the M.abscessus complex esp. those resistant to existing standard of care (Clarithromycin and Macrolides) and antimicrobials to Mycobacterium avium.
Antimicrobials focused on treating infections associated with other emerging pathogens associated with CF; Strenotrophomonas maltophilia, H.influenzae, B.cepacia (and related species such as B.cenocepacia, B. multivorans), Achromobacter spp. Pandoraea spp, Ralstonia spp, S. aureus. Treatments to fungal pathogens associated with chronic infections in CF (Aspergillus, Candida albicans) were also considered in scope.
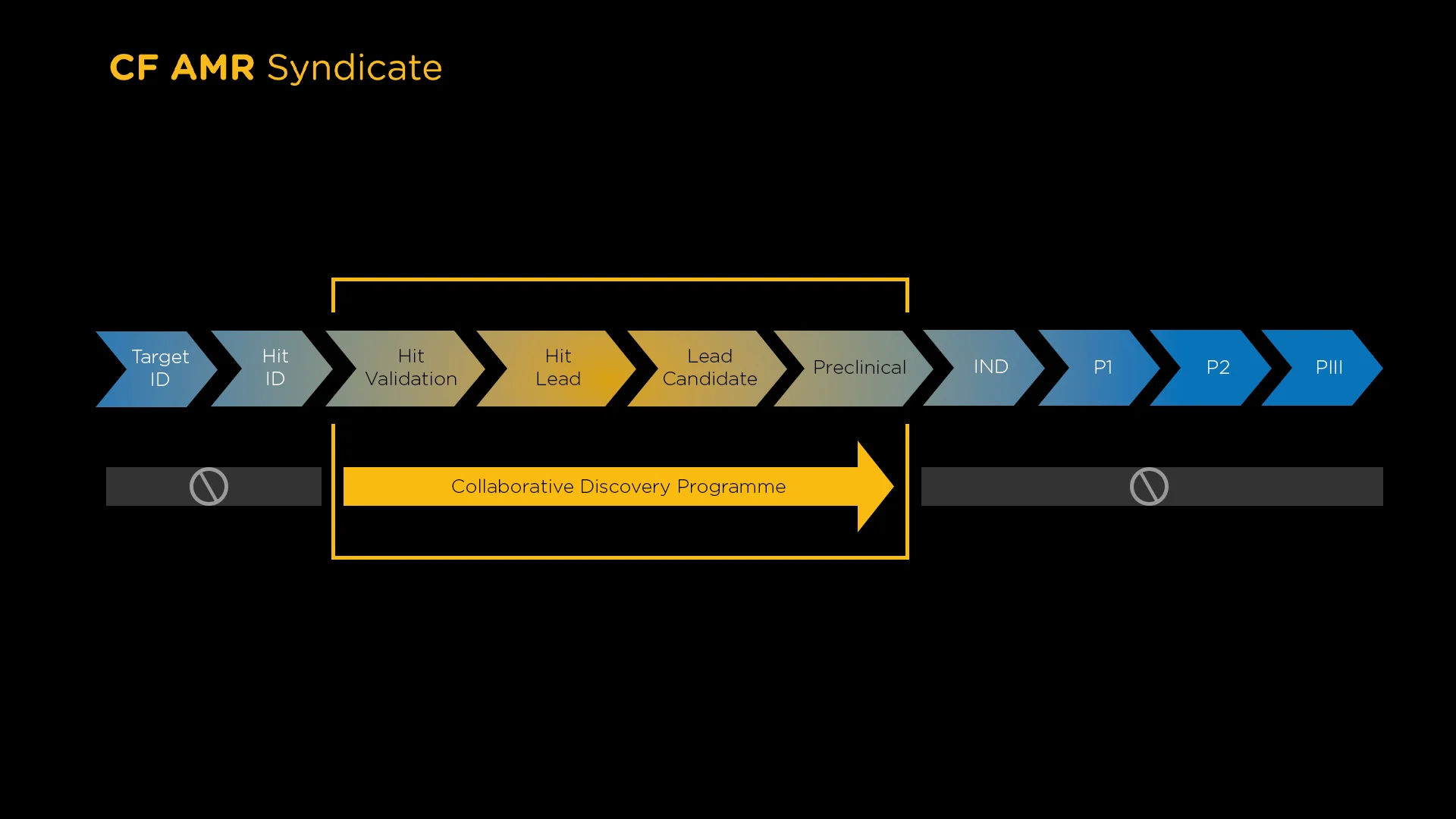
Grant funding was open to researchers in academia and small and medium enterprises (SMEs) worldwide and projects were required to be early phase from hit validation through lead optimisation and early preclinical. Varying modalities were considered e.g. small and large molecule, phage etc, either direct-acting and otherwise (e.g. anti-virulence).
The 2023 funding call sought to identify projects aligned to our therapeutic TPPs
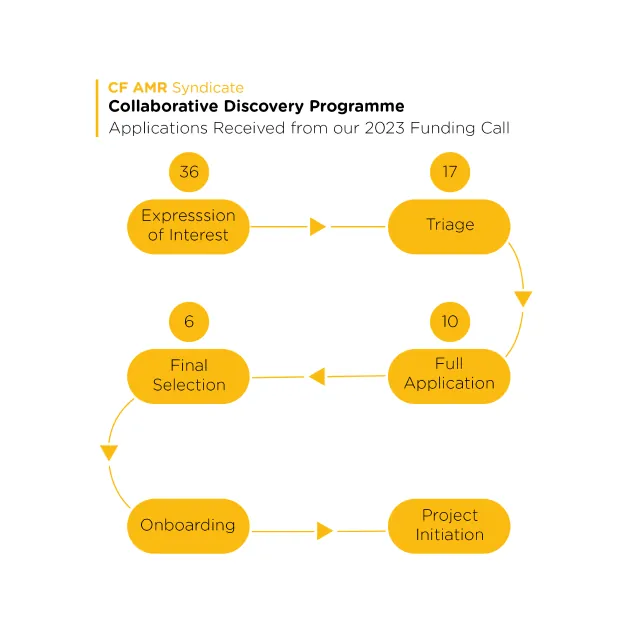
Applications Received
Following the launch of CDP and close of the expression of interest the CF AMR Syndicate received 36 expressions of interest from academics and small medium enterprises globally. Out of the expressions of interest, 17 were selected for full stage review (based on scope and criteria), following panel selection 10 applicants were invited to submit full applications. Incubator support happened right at the start of the launch of the call.
Applicants were provided with support and advice by the CF AMR Syndicate throughout all stages of the application process via 1-1 meetings, workshops and webinars. Engagement and input from people with lived experience of CF was a key part of the review process.
The 36 expressions of interest received were from 11 countries across the globe and encompassed a broad range of therapeutic modalities.
Scientific Advisory Board
To enhance the support of the CDP portfolio each awardee within the portfolio has access to a bespoke scientific advisory board. To ensure the highest standards are attained, our scientific advisory board is comprised of subject matter experts with the relevant knowledge and expertise to provide the strategic guidance required to deliver the CDP portfolio of projects. Meet the advisory board:
Pam Brown
Pam has over 35 years of experience in antibacterial drug discovery within both the large Pharma and biotech sector.
Lloyd Czaplewski
Chief Scientific Officer for Persica Pharmaceuticals Limited, a Director of Chemical Biology Ventures Ltd, which provides life & chemical sciences consultancy services and is a Non-Executive Director at Curza.
Shampa Das
Shampa Das (BSc, PhD) is a Professor of Antimicrobial Therapeutics at the University of Liverpool and PK/PD consultant.
David Nichols
David Nichols, MD is Senior Director of Clinical Research Development at the CF Foundation and Affiliate Professor of Pediatrics at the University of Washington.
Deborah O’Neil
A biotechnology entrepreneur and immunologist by training, Deborah has over two decades of experience in drug discovery and development.
Peter Warn
Peter Warn is a consultant at Magic Bullet Consulting with expertise in the development of antimicrobial agents.