The unmet need
CF is an inherited, life-limiting disease affecting over 11,300 people in the UK and approximately 188,000 people globally.
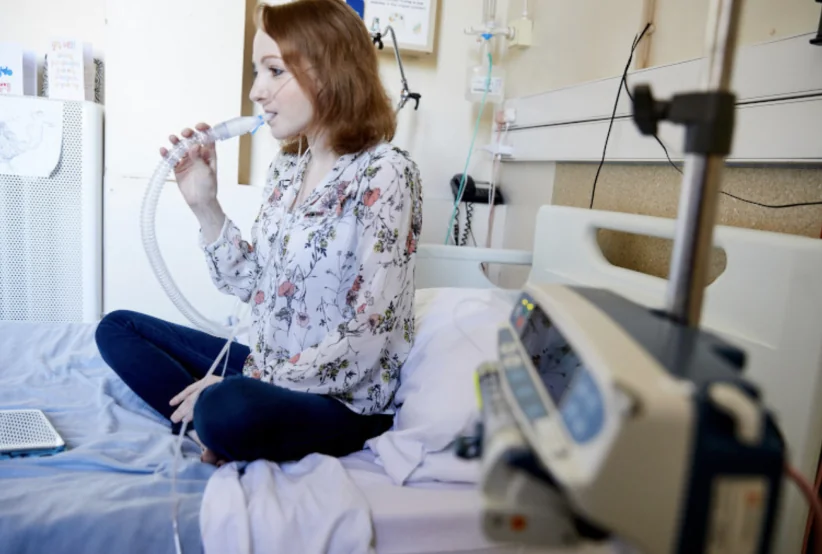
People with CF experience a buildup of thick, sticky mucus in the lungs, digestive system, and other organs. This causes a wide range of challenging symptoms affecting the entire body. In the lungs, thick sticky mucus traps bacteria, causing persistent and resistant infections that drive lung damage.

Persistent and resistant infections, such as those caused by Pseudomonas aeruginosa and nontuberculous mycobacteria (NTM), continue to threaten lung function and quality of life.
Current diagnostic tools often fail to detect infections early or accurately, delaying treatment and leading to chronic infection or disease progression. The availability of CFTR modulator therapies has changed the clinical landscape, but many people with CF report changes in exacerbation symptoms, complicating clinical management. The burden of care remains significant, with limited new antimicrobial options and increasing antibiotic resistance adding to the challenges faced by people living with CF.
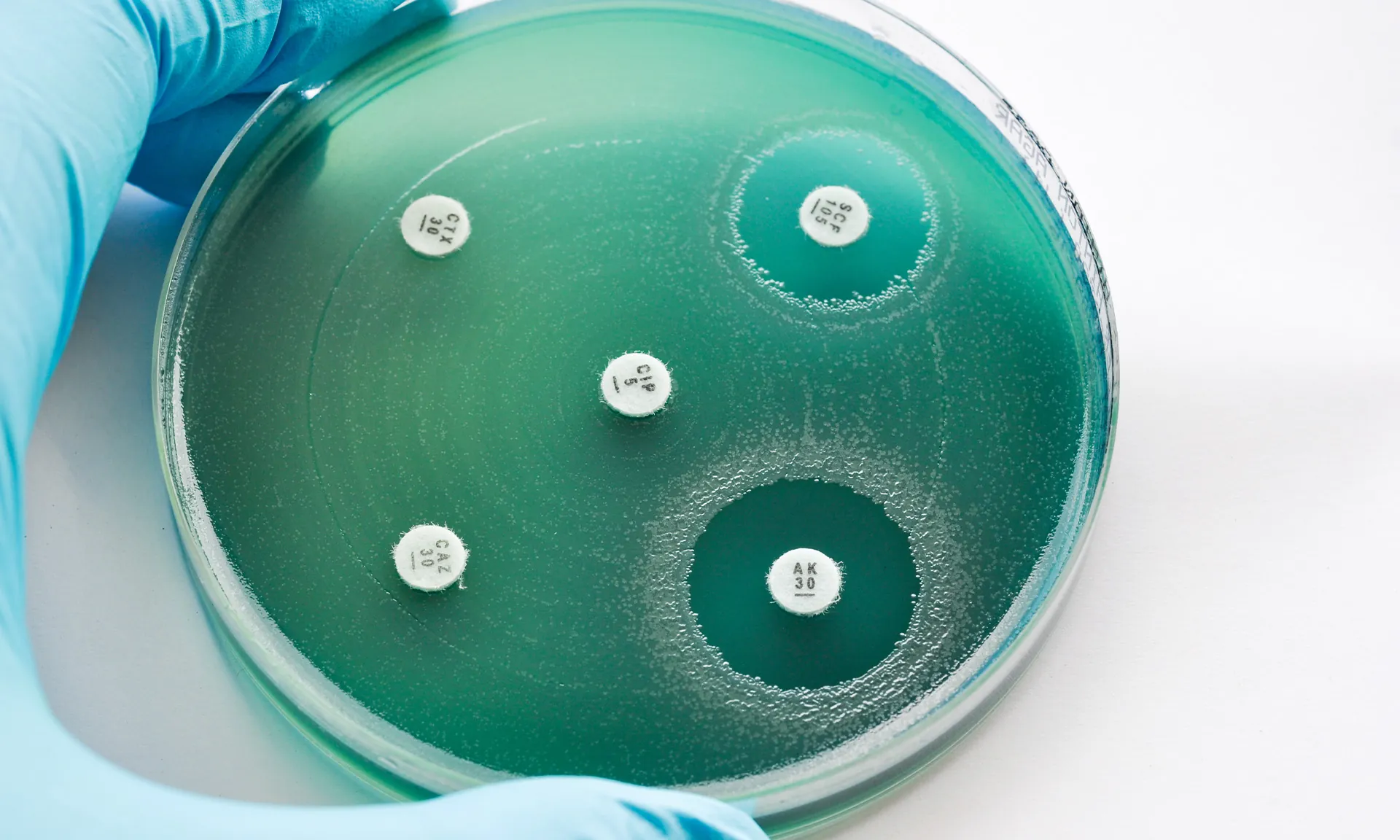
Antimicrobials are vital to managing acute infections, known as exacerbations, and to help suppress or manage long-term chronic infections. Without these treatments, lung function and overall health, quality of life and the life span of people living with CF are dramatically altered.
The discovery and development of new antimicrobials and diagnostics to treat and manage infections associated with CF are, undoubtedly, an urgent unmet need.

The Challenge for Innovators
CF infections are a challenging indication for industry. There is limited regulatory guidance, and few SMEs work in this space. Navigating the complex discovery and regulatory landscape requires insight and expertise that is not easy for many innovators to access. This means that they may not know where the best opportunities lie.
Despite global activity in CF drug discovery and diagnostic development, key challenges affecting researchers include difficulties accessing clinically relevant samples and data, lack of agreed roadmaps and target product profiles for CF antimicrobials and diagnostics, and in some cases lack of access to appropriately validated preclinical models and tools to predict clinical effect. Early-stage funding gaps further compound these barriers.
CF antimicrobial and diagnostic developers are typically academic groups or SMEs who need access to both funding and support to bridge the early translational research gap, developing de-risked assets attractive for onward investment. Without coordinated support, translation remains slow and high-risk, making it difficult for innovators to progress their programmes effectively.

Our Approach
The CF AMR Syndicate has been established to accelerate the translation of CF antimicrobials and diagnostics to the clinic to bring new and effective treatment options to people with CF.
Our research agenda has been informed by extensive efforts to understand the research landscape and the needs of the CF infection research community. We have developed the agenda through a cross-sector collaborative approach, which has involved close working with people with CF.
The CF AMR Syndicate, managed by Medicines Discovery Catapult, Cystic Fibrosis Trust, and LifeArc, connects a coordinated cross-sector community to overcome these barriers by generating insights, understanding opportunities, and developing new capabilities that create the right conditions for innovation
Value for Innovators
Innovators can engage with the CF AMR Syndicate to access expert guidance, insight-driven resources, and a coordinated network spanning the entire discovery-to-clinic pathway. By working with us, innovators can use CF as a strategic testbed to validate their discoveries, de-risk their development programmes, and generate solutions with broader impact for other chronic respiratory infections.

We support the successful translation of discoveries into therapeutics and diagnostics through:
Insight-Driven Resources
Target Product Profiles (TPPs): Patient-focused profiles for CF-related infections developed through network engagement to guide discovery and development efforts aligned with real-world needs.
UK CF Infection Biorepository: High-quality samples and data, including CF-relevant strains and isolates, enabling innovators to test treatment effectiveness.
Collaborative Network
Building a CF AMR network to facilitate collaboration and enable knowledge exchange across academia, industry, clinical teams, and people with CF, supporting translational research efforts.
Project Support
Collaborative Discovery Programme: Grant funding and wraparound support for early-stage drug discovery projects aligned with real world needs identified in our TPPss, advancing antimicrobial development.
Dedicated support through Medicines Discovery Catapult, Cystic Fibrosis Trust, and LifeArc.
Our History
Contact CF AMR Syndicate
Have a question you can’t find the answer to or would like further information?
Thinking of joining our network?
Become part of a collaborative community that is working together to accelerate the development of CF antimicrobials and diagnostics and bring new treatments to people with CF.