Collaborative Discovery Programme
Advancing Preclinical Phase Projects for Cystic Fibrosis Lung Infections
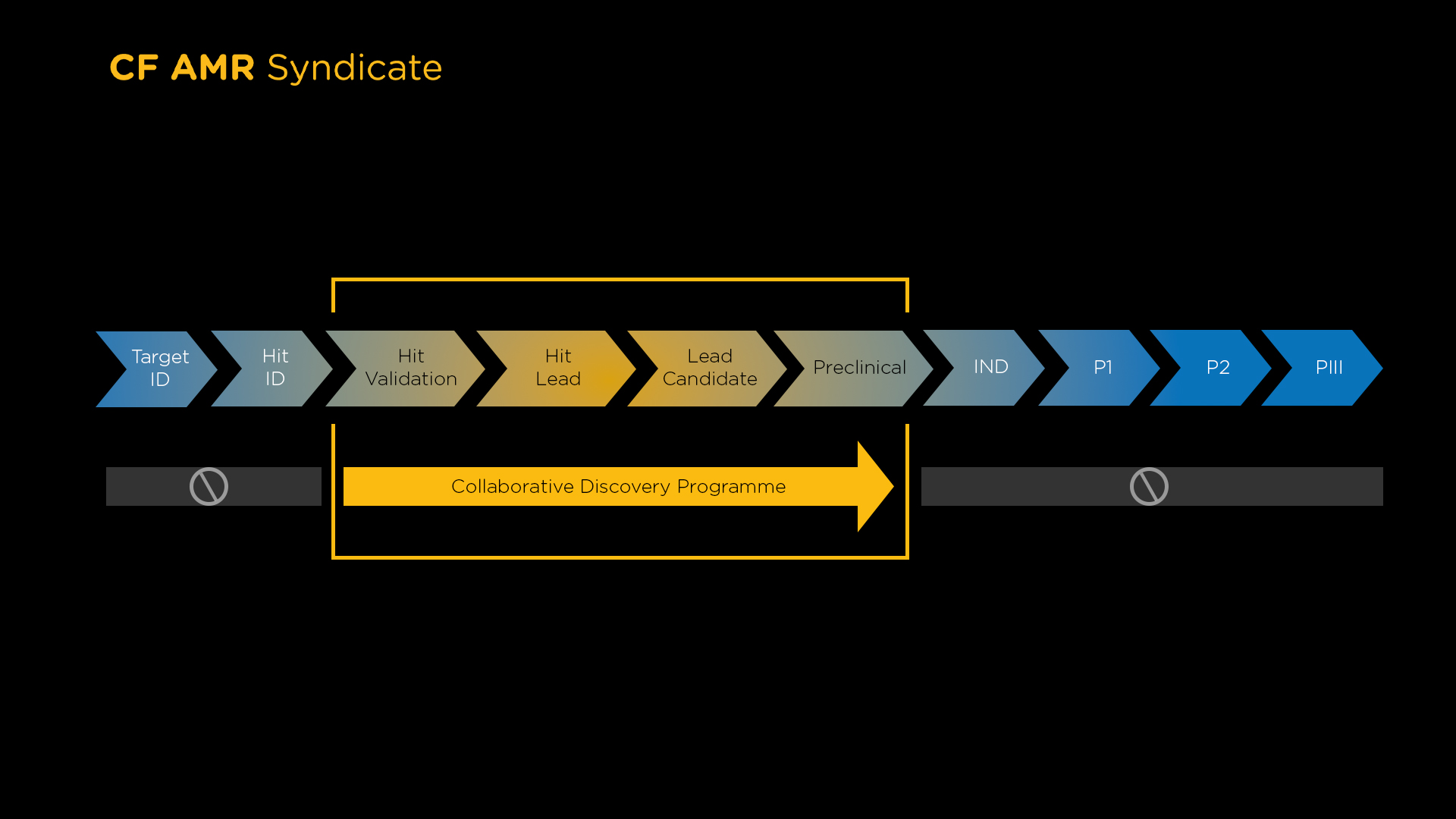
The CF AMR Syndicate Collaborative Discovery Programme (CDP) is advancing an exciting and diverse pipeline of preclinical phase projects for Cystic Fibrosis (CF) lung infections. Our £3 million (GBP) programme (provided by LifeArc) is supporting six early-stage drug discovery projects aligned with the unmet needs identified in our Therapeutic Target Product Profiles (TPP) for CF lung infections.
CDP offers a unique collaborative approach to project development and delivery. As well as funding, our awardees benefit from additional collaborative support, increasing the probability of successful outcomes.
Support through the early development phases to generate essential data packages will position these projects to attract onward funding and investment for further development.

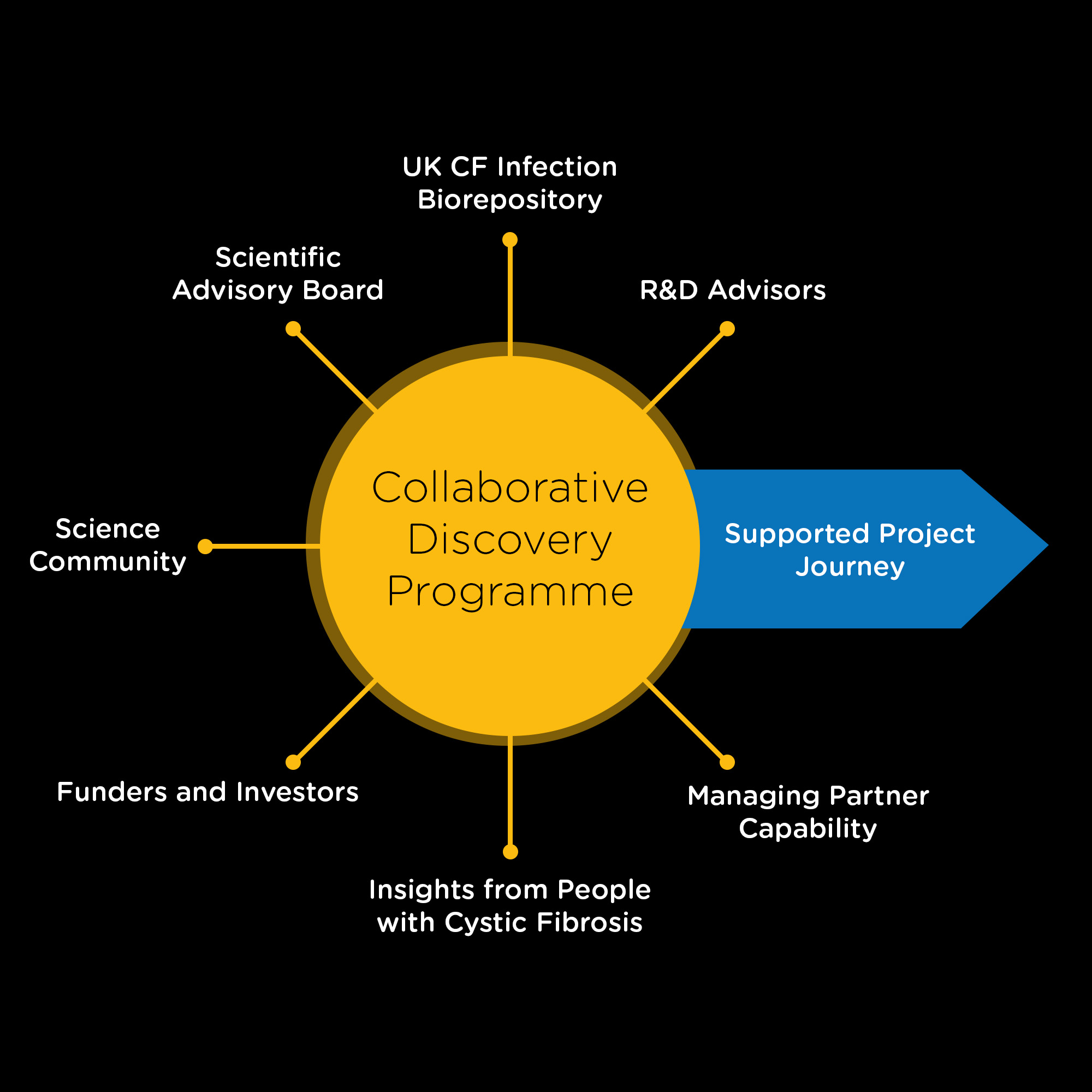
More than just Funding
Collaboration at the Centre
- – Collaborative support – overcoming barriers together
- – Provision/access to CF AMR Syndicate resources
- – Drug discovery and disease area insight and advice
- – Support to develop actionable project plans aligned to TPP
Our Project Pipeline
Project Summary
BioVersys’ Non-tubercular Mycobacteria (NTM) preclinical program is derived from the company’s proprietary Ansamycin Chemistry platform. The BioVersys’ research team is developing a novel and highly potent best in class, broad-spectrum anti-NTM ansamycin, suitable for oral or inhalation therapy that is devoid of cross-resistance with other therapeutic classes and does not show any significant potential for drug-drug interactions.
Company Summary
BioVersys AG is a multi-asset, clinical stage biopharmaceutical company focused on identifying, developing and commercializing novel antibacterial products for serious life-threatening infections caused by multi-drug resistant (“MDR”) bacteria. Derived from the company’s two internal technology platforms (TRIC and Ansamycin Chemistry), candidates are designed and developed to overcome resistance mechanisms, block virulence production and directly affect the pathogenesis of harmful bacteria towards the identification of new treatment options in the antimicrobial and microbiome fields. This enables BioVersys to address the high unmet medical need for new treatments against life-threatening resistant bacterial infections and bacteria-exacerbated chronic inflammatory microbiome disorders. The company’s most advanced research and development programs address nosocomial infections of Acinetobacter baumannii (BV100, Phase 2), and tuberculosis (alpibectir, Phase 2a, in collaboration with GlaxoSmithKline (GSK) and a consortium of the University of Lille, France). BioVersys is located in the biotech hub of Basel, Switzerland.
More on the Call Scope
This first funding call sought to identify project proposals with the potential to address needs identified in our therapeutic TPPs in particular:
- Antimicrobials focused on Pseudomonas aeruginosa infections as 1) a maintenance therapy to suppress chronic respiratory infection or 2) treatment of acute pulmonary exacerbations linked to Pseudomonas aeruginosa infection.
- Antimicrobials focused on Mycobacterium abscessus infections as 1) a maintenance therapy to suppress chronic respiratory infection or 2) treatment of acute pulmonary exacerbations linked to M.abscessus infection or 3) eradication treatment. Also in scope, treatments targeting ‘fast-growing’ NTMs of the M.abscessus complex esp. those resistant to existing standard of care (Clarithromycin and Macrolides) and antimicrobials to Mycobacterium avium.
- Antimicrobials focused on treating infections associated with other emerging pathogens associated with CF; Strenotrophomonas maltophilia, H.influenzae, B.cepacia (and related species such as B.cenocepacia, B. multivorans), Achromobacter spp. Pandoraea spp, Ralstonia spp, S. aureus. Treatments to fungal pathogens associated with chronic infections in CF (Aspergillus, Candida albicans) were also considered in scope.
Grant funding was open to researchers in academia and small and medium enterprises (SMEs) worldwide and projects were required to be early phase from hit validation through lead optimisation and early preclinical. Varying modalities were considered e.g. small and large molecule, phage etc, either direct-acting and otherwise (e.g. anti-virulence).
The 2023 funding call sought to identify projects aligned to our therapeutic TPPs
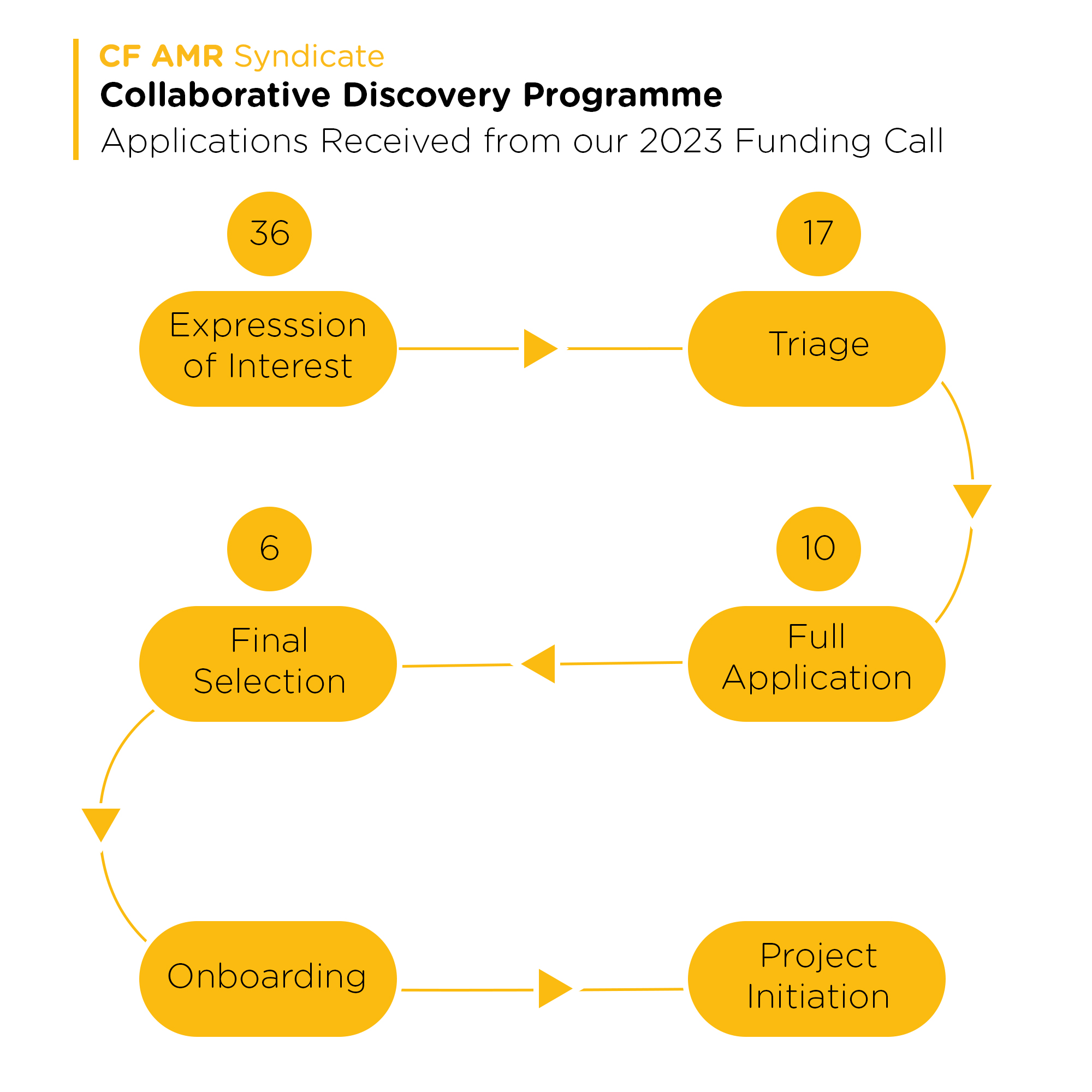
Applications Received
Following the launch of CDP and close of the expression of interest the CF AMR Syndicate received 36 expressions of interest from academics and small medium enterprises globally. 17 were selected for full stage review (based on scope and criteria), following panel selection 10 applicants were invited to submit full applications.
Incubator support happened right at the start of the launch of the call…
Applicants were provided with support and advice by the CF AMR Syndicate throughout all stages of the application process via 1-1 meetings, workshops and webinars. Engagement and input from people with lived experience of CF was a key part of the review process.
Breakdown of applications
The 36 expressions of interest received were from 11 countries across the globe and encompassed a broad range of therapeutic modalities.
Scientific Advisory Board
To enhance the support of the CDP portfolio each awardee within the portfolio has access to a bespoke scientific advisory board. To ensure the highest standards are attained, our scientific advisory board is comprised of subject matter experts with the relevant knowledge and expertise to provide the strategic guidance required to deliver the CDP portfolio of projects. Meet the advisory board:












